Immunocard STAT!® EHEC
Rapid lateral flow immunoassay for the detection of Shiga toxin 1 and 2 produced by enterohemorraghic E. coli (EHEC) from stool specimens in broth enrichment.
NOTE: Actual product may vary from image displayed. Please refer to the package insert for complete information.
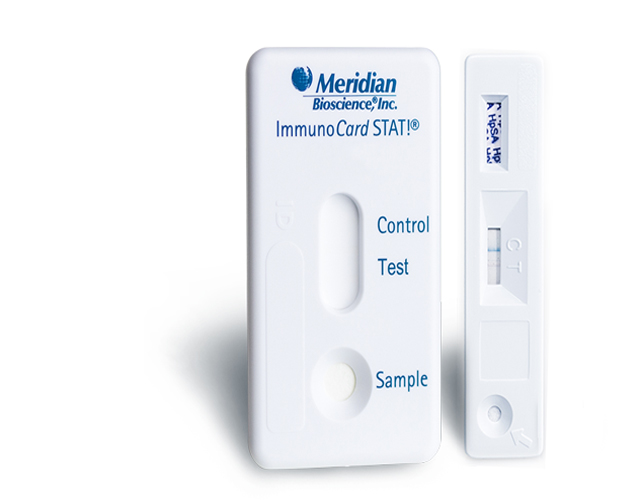
Definitive answers, confidence in results
Related Tests
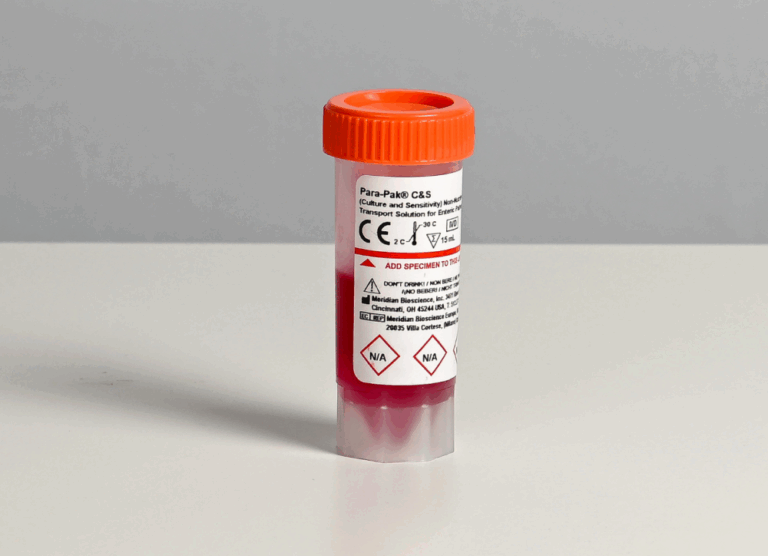
Para-Pak
The Para-Pak system provides a standardized procedure for the routine collection, transport, preservation, and culture of stool specimens for bacterial enteric pathogens. Learn More.
Related Products
Immunocard STAT!® CAMPY
C&S Stool Transport
Clean Stool Transport

Support & Documents
Downloadable PDFs
Webinars & Videos
FAQs
Does Immunocard STAT! EHEC only test for E. coli O157 strains?
Does this test require a broth enrichment step?
Yes, CDC Guidelines recommend Shiga Toxin testing be performed on growth from broth culture because this method is more sensitive and specific than direct testing of stool. In addition, because the amount of free fecal Shiga toxin in stools is often low, EIA testing of broth enrichments from stools is recommended rather than direct testing.
Why use a Shiga Toxin test for the non-O157 strains?
It is known that culture will miss ALL non-O157 strains. It is also known that the sensitivity for culture detecting the O-157 strains is low. The documented infection rate is about 50:50 for O157 strains vs. non-O157 strains. You should be testing for both!
References:
- https://www.nphl.org/documents/ShigaToxinTestingRecommendations.pdf
- CDC Recommendations and Reports MMWR October 16, 2009 / 58(RR12);1-14
- Morbidity and Mortality Weekly Report. 2013;62(50):1029-1031.
What is the suggested CPT Code?
Immunocard STAT!® EHEC – STX I 87899 , STX II 87899-59
Broth Enrichment – 87015
Pricing
- Email: sales@meridianbioscience.com
- Phone: +1 800 696-0739
- International Sales Extension: +1 513 271-3000
